It is clear that coronavirus vaccines are safe and effective, but as more are rolled out, researchers are learning about the extent and nature of side effects.
As people around the world receive COVID-19 vaccines, reports of temporary side effects such as headaches and fevers are rolling in. Much of this was expected — clinical-trial data for the vaccines authorized so far suggested as much. But now that millions of people are vaccinated, compared with the thousands enrolled in early studies, reports of some rare, allergic reactions are surfacing, and questions are arising about whether any deaths are linked to the shots.
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly coronavirus. Nature looks at what scientists are learning about the frequency and nature of side effects as huge numbers of people report their reactions to physicians and through safety-monitoring systems, such as smartphone apps.
How many people experience common side effects from COVID-19 vaccines?
For the two available messenger RNA (mRNA) vaccines — one made by Moderna at Cambridge, Massachusetts, and the other developed through a collaboration between Pfizer in New York City and BioNTech in Mainz, Germany — a significant portion of people experience non-serious reactions, such as injection-site pain, headache and fatigue. These vaccines deliver bits of RNA that code for coronavirus proteins, which the body mounts a response against.
According to data from the US Vaccine Adverse Event Reporting System (VAERS), about 372 out of every million administered doses of the mRNA vaccines lead to a non-serious reaction report. This number is lower than would be expected from clinical-trial data, which indicated that at least 80% of people would experience injection-site pain. Researchers running trials monitor patients closely and record every reaction. VAERS, meanwhile, relies on health-care workers and vaccinated individuals to self-report side effects.
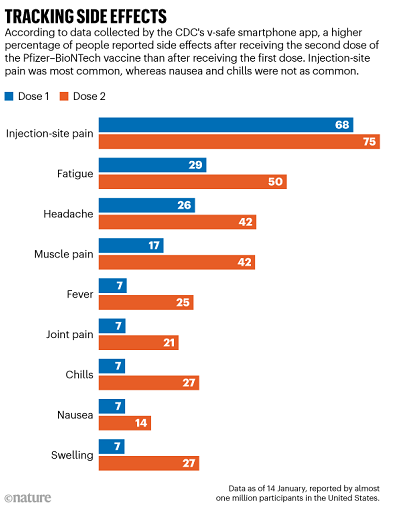 So far, reactions to the mRNA vaccines are similar. These vaccines are administered in a two-dose regimen: the first shot triggers an immune reaction, and the second is a ‘booster’ that strengthens the body’s ability to fight the coronavirus. For the Pfizer–BioNTech vaccine, which has been in use longer than the Moderna vaccine and therefore has generated more data, side effects increase with the second dose (see ‘Tracking side effects’).
So far, reactions to the mRNA vaccines are similar. These vaccines are administered in a two-dose regimen: the first shot triggers an immune reaction, and the second is a ‘booster’ that strengthens the body’s ability to fight the coronavirus. For the Pfizer–BioNTech vaccine, which has been in use longer than the Moderna vaccine and therefore has generated more data, side effects increase with the second dose (see ‘Tracking side effects’).
In the United Kingdom, three million doses of another vaccine, developed by the University of Oxford and pharmaceutical firm AstraZeneca, have been doled out. This vaccine, which also requires a two-dose regimen, contains a inactivated cold-causing adenovirus with genetic instructions for making coronavirus proteins to trigger immunity. According to UK safety-monitoring system the Yellow Card Scheme, about 4,000 doses out of every million administered lead to adverse reactions. Again, clinical-trial data suggest that a higher frequency is more accurate: around 50% of participants had injection-site pain, headache or fatigue, according to data reported to the European Medicines Agency (EMA).
Few people have received a second dose of the Oxford–AstraZeneca vaccine because the United Kingdom used its supplies to administer a first dose to as many people as possible, but clinical-trial data presented to the EMA suggest that side effects of the second shot are milder than those caused by the first.
Safety data for shots rolling out in other parts of the world, such as the COVID-19 vaccines in China, are harder to come by. Preliminary data from clinical trials of the adenovirus-based Sputnik V vaccine in Russia suggest its most common side effects include flu-like symptoms and injection-site reactions1.
How does that compare with side effects from an annual flu shot?
At least for the mRNA vaccines, physicians are seeing more side effects than for flu shots, says Helen Chu, an infectious-disease specialist at the University of Washington School of Medicine in Seattle, who directs the Seattle Flu Study. In clinical trials for the Pfizer–BioNTech vaccine, for instance, 75% of participants reported a ‘systemic reaction’, such as headache, fever or chills. In a clinical trial for the common influenza vaccine Flubok Quadravalent, around 34% of participants aged 18–49 had a systemic reaction. Side effects were even less frequent in study participants who were at least 50 years old.
Chu says the mRNA COVID-19 vaccines generate a particularly strong immune response that increases the risk of side effects, although this also means that the vaccines are working. She notes that her second dose of the Pfizer–BioNTech vaccine made her ill. “I got the vaccine, and 6 hours later, I had chills, a high fever, muscle aches and I went to bed for 24 hours,” she says. “Then by 36 hours later, it was totally over and I was back to normal.” But Chu would rather be temporarily ill from a vaccine than deal with COVID-19, “a potentially mortal disease that could kill me”, she says.
Have investigations linked any deaths to a COVID-19 vaccine?
Although some have questioned whether the vaccines have led to deaths, none have been directly attributed to a COVID-19 jab. After 33 elderly care-home residents in Norway died within 6 days of receiving the Pfizer–BioNTech vaccine, investigations by both the Norwegian Medicines Agency and the World Health Organization concluded that these deaths were in line with normal death rates in this age group and that the vaccine is still safe for older people. India’s Ministry of Health and Family Welfare reported 27 deaths in the country, but none of these have been linked directly to a COVID-19 vaccine either.
It is “extremely difficult” to definitively link a death to the vaccine itself, says Hilda Bastian, a writer and scientist who specializes in validating evidence-based health claims. That is partially because the deaths reported so far have occurred days or weeks after an injection, making it hard to rule out other circumstances. Another reason is that, right now, clinicians are prioritizing vaccines largely for a population of older people with underlying health conditions. Most of those who have died after vaccination have been in this group, according to reports from the United Kingdom and the United States.
What do researchers know about the rare, but severe, allergic reactions to the vaccines?
The Moderna vaccine elicits about three anaphylactic reactions per million doses administered, and the Pfizer–BioNTech vaccine triggers five reactions per million doses, according to VAERS data. This is a higher rate than most other vaccines — including annual flu shots, which trigger anaphylaxis for only one out of every million doses administered2. For the Oxford–AstraZeneca vaccine, 30 cases of anaphylaxis have been confirmed overall so far, out of a little more than 3 million administered doses. Vaccine specialists expect that these rates might change as more shots are administered.
Although some people have required hospitalization, all have fully recovered. Public-health officials advise people with a history of allergies to any of the vaccines’ ingredients not to get a COVID-19 jab.
Unlike COVID-19, anaphylaxis is treatable with drugs such as epinephrine if caught quickly, says Paul Offit, a vaccine and infectious-disease specialist at the Children’s Hospital of Philadelphia in Pennsylvania, who participated in the US Food and Drug Administration advisory-committee meetings that led the agency to authorize both mRNA vaccines. “I wish that SARS-CoV-2 could be immediately treated with a shot of epinephrine!” he says.
Most of the people who experienced anaphylaxis had reacted to other substances before: about 80% of people who reacted to the Pfizer–BioNTech vaccine, and 86% to the Moderna vaccine, had a history of allergies, according to the US Centers for Disease Control and Prevention.
The specific cause of the anaphylactic reactions remains unknown, but the US National Institute of Allergy and Infectious Diseases told Nature in an e-mail that the agency has designed a clinical trial to determine the underlying mechanism, but did not specify when the trial would begin.
What could be causing the allergic reactions?
Some researchers have had their eye on polyethylene glycol (PEG) as the anaphylaxis-causing agent in the mRNA vaccines. The Moderna and Pfizer–BioNTech vaccines use hollow lipid nanoparticles to store and then deliver their mRNA payload to cells. PEG is linked to the lipids in these particles and, under normal circumstances, helps them to sneak by the immune system. Although PEG-linked molecules are found in a variety of products, such as laxatives and gout medicines, they have been known to cause allergic reactions3.
Follow-up studies in people who experienced anaphylaxis could help to determine whether PEG is the culprit, says Samuel Lai, a pharmaco-engineer at the University of North Carolina at Chapel Hill. If blood samples from these people contain anti-PEG antibodies, it could be an indicator, says Lai, but it is as yet unclear how long these proteins remain in the bloodstream after anaphylaxis.
Vaccines that don’t use PEG — such as the not-yet-authorized shot from Johnson & Johnson, which also uses an adenovirus to trigger immunity to the coronavirus — might be a way to vaccinate people with a sensitivity to the polymer, he adds.
Because mRNA vaccines have shown such promise, Ulrich Schubert, a polymer scientist at the University of Jena in Germany, thinks now is the time to invest in developing vaccine-compatible polymers that don’t cause allergic reactions. At the German Research Foundation-funded collaborative research center PolyTarget, where Schubert works, these studies are already in progress. “If we want to be ready for the next pandemic — which will come — we have to start now,” he says.